Product Details
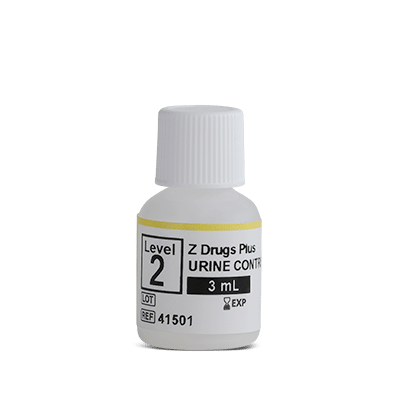
The UTAK Z Drugs panel Level 2 Control is used as a qulity contorl material measuring the levels of analytes in urine.
Product Contains: (±)-Baclofen, Dextromethorphan, Dextrorphan, Gabapentin, Ketamine, Norketamine, Lamotrigine, Mitragynine, Naloxone, Naltrexone,6-β-Naltrexol, Pregabalin, Topiramate, Zaleplon, Zopiclone, Zolpidem, Zolpidem Phenyl-4-Carboxylic Acid
Matrix: Urine
Unit Size: 5x3mL
Form: Lyophilized
Shelf Life: 30 months
Storage: Store dried control material at 2-8℃ (35-46℉).